LAUNCH ALERT: FDA Approves YCANTH for Molluscum Contagiosum
By Dermsquared Editorial Team | July 25, 2023
Watch here as James Del Rosso, DO, discusses the unique features of YCANTH, explains the application process, and remarks on the benefits and importance of this first-ever FDA-approved treatment for molluscum contagiosum.
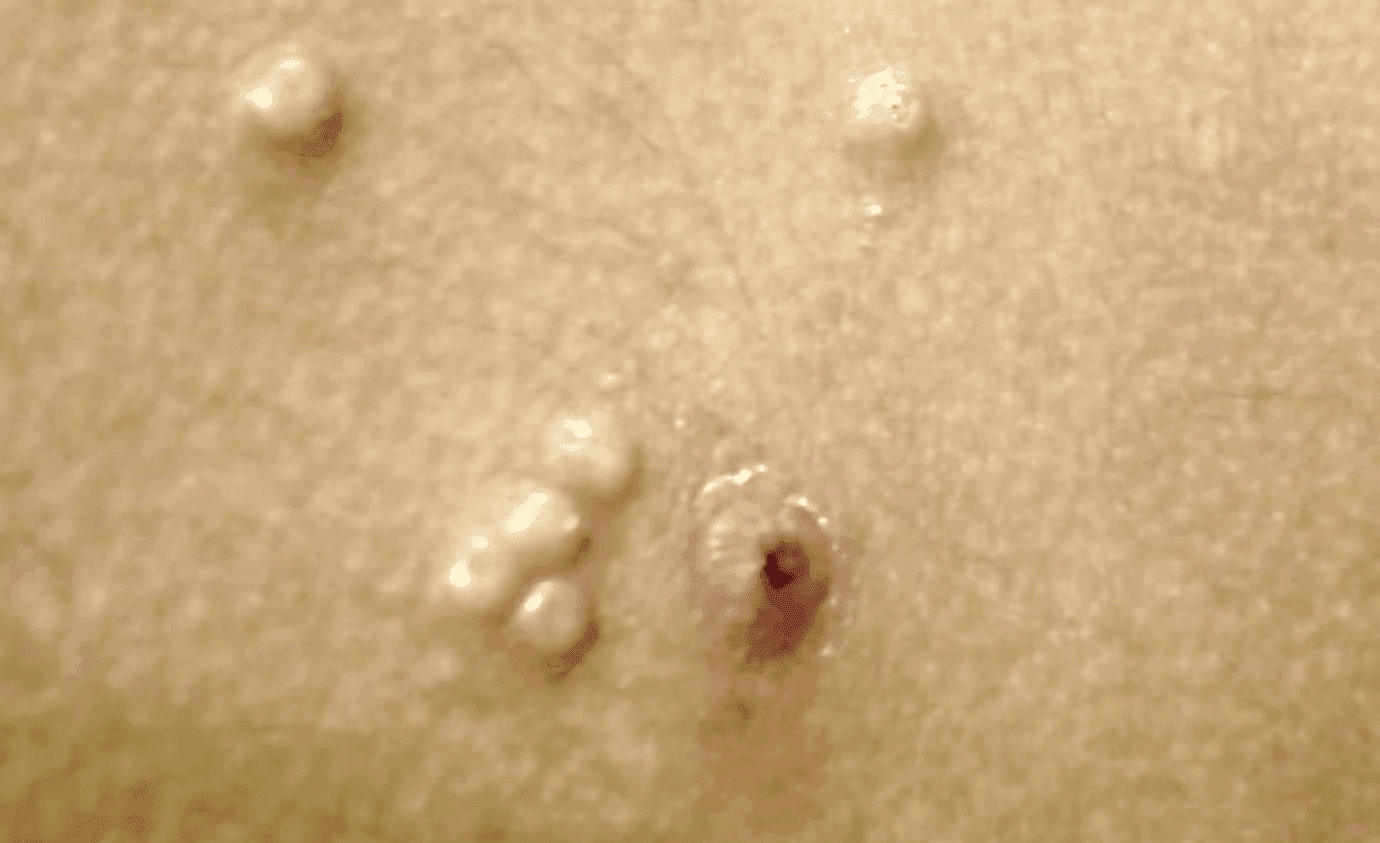
Verrica Pharmaceuticals has announced the launch of YCANTH, the first and only FDA-approved treatment for molluscum contagiosum in adult and pediatric patients 2 years and older.
YCANTH is a drug-device combination product containing a GMP-controlled formulation of cantharidin (0.7% w/v). YCANTH is delivered via a single-use applicator and applied directly to lesions with a specialized applicator tip, allowing for precise topical administration.
FDA approval was issued after positive outcomes from the CAMP-1 and CAMP-2 trials, which assessed the safety and effectiveness of VP-102 (YCANTH) in patients 2 years and older who were diagnosed with molluscum contagiosum. In the identical phase 3 randomized, double-blind, multicenter trials, a clinically and statistically significant number of patients treated with VP-102 achieved the primary endpoint, which was complete clearance of all treatable lesions.
In CAMP-1, 46% of participants receiving VP-102 achieved complete clearance of molluscum lesions versus 18% in the placebo group. Similarly, in CAMP-2, 54% of participants receiving VP-102 achieved complete clearance compared with 13% in the placebo group.
No serious adverse reactions were reported in the trials. The most common mild-to-moderate adverse reactions (incidence ≥1%) reported were vesicles, pain, pruritus, scab, erythema, discoloration, dryness, edema, erosion, and contact dermatitis.
Verrica plans to make YCANTH available commercially in September 2023.